3 Things You Need to Know About Microbiology for Mine Water Treatment
Many types of water treatment rely on biological or biogeochemical reactions. This includes biochemical reactors (also called bioreactors) and treatment wetlands, and also in situ treatment of saturated rock fills, pit lakes, underground mine pools, and tailings ponds. Those reactions are done by microbes. Decades ago, biological systems were viewed as a “black box”, this is no longer the case with advanced genomics techniques. This post is designed to provide an introduction to a few of the microbiology and biogeochemical processes that are done by microbes in water treatment.
If you’d like more information about any of the topics below, please contact us, or comment below!
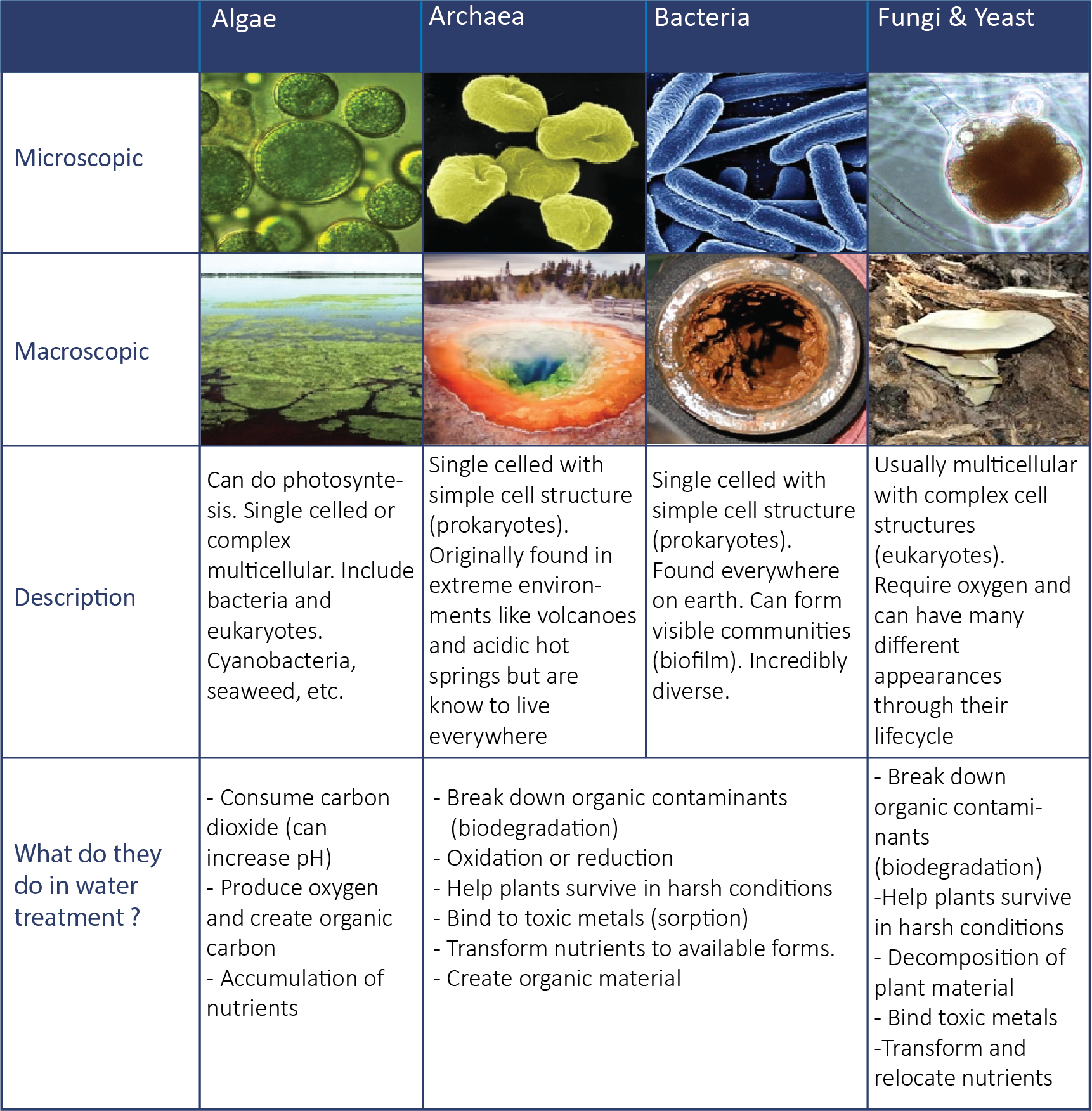
1. There are many types of microbes
2. “Carbon Source” means something important to microbes biological water treatment
Microbes need energy to grow and also to perform the reactions that treat contaminants in water. Some organisms can get their energy from the sun (autotrophs), some can use electrons from metals (chemolithotrophs), but a large percentage of organisms get their energy from organic carbon sources (e.g., plant material). These organisms get their energy by breaking carbon sources apart, releasing stored electrons. Bonds between carbon molecules contain energy that microbes use as the carbon source is released as smaller carbon compounds, carbon dioxide and water.
Table 1. Complexity of carbon sources used in biological and semi-passive water treatment
In semi-passive treatment, carbon sources can range from complex materials such as wood chips and plant material, to refined materials such as ethanol, methanol, and glycerol. Just as humans require “specialists” to prepare and make certain foods accessible for us to easily consume (e.g., processing a cow to a steak), microbial communities also require specialists to process complex carbons (Figure 1 and Figure 2). Only some microbes will be able to break down complex carbon sources (e.g., wood chips, cellulose), while most will be able to use “simple” carbon sources like sucrose and glucose. However, some ‘simple’ carbon sources such as ethanol and glycerol can only be used by specific organisms, making it possible to tailor designs. Additionally, as the carbon source breaks down, the total stored energy that can be gained by the microbes decreases (Figure 2).
Figure 1. Comparison of beef processing to microbial processing of complex carbon sources
Figure 2. Overview of microbial carbon utilization pathways and energy yield
3. Microbes play many predictable roles in biological semi-passive water treatment
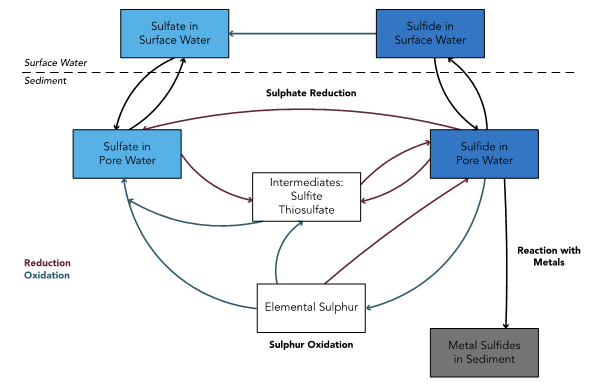
Microbes transform constituents in water using both reductive and oxidative processes. This means they use constituents (including some contaminants) as electron donors or acceptors and transform them into less bioavailable forms. For example, microbes can help remove dissolved metals from water by reducing sulphate and elemental sulfur in water to sulphides, which form insoluble metal sulphides that precipitate out of the water (Table 2, Figure 4). These equations are understood through stoichiometry (the math of chemistry).
Table 2. Examples of contaminants treated through reduction and oxidation
Figure 3. Microbially mediated processes in the nitrogen cycle
Oxidizing reactions (AOB and NOB) are using ammonia and Nitrite as a source of electrons. Reducing reactions like denitrification means they are using the nitrogen compound as an electron acceptor in a reaction where they are taking the energy from another compound.
Figure 4. Microbial processes in the sulfur cycle
Microbes facilitate the transformation of Sulphur from soluble, reactive sulfide to insoluble elemental sulfur, intermediate compounds and sulfate.