Oxidation-Reduction Reactions for Mine Water Treatment
In a previous post, we discussed transfers and transformations as the two main ways to treat contaminants from water. One of the key types of “transformations” is oxidation-reduction processes. Oxidation reduction potential is often referred to as ORP or as “redox” (short for reduction-oxidation). This is a reaction that can be done chemically (for example, peroxide can oxidize cyanide) or assisted by microbes (microbes can oxidize ammonia).
What Is Redox?
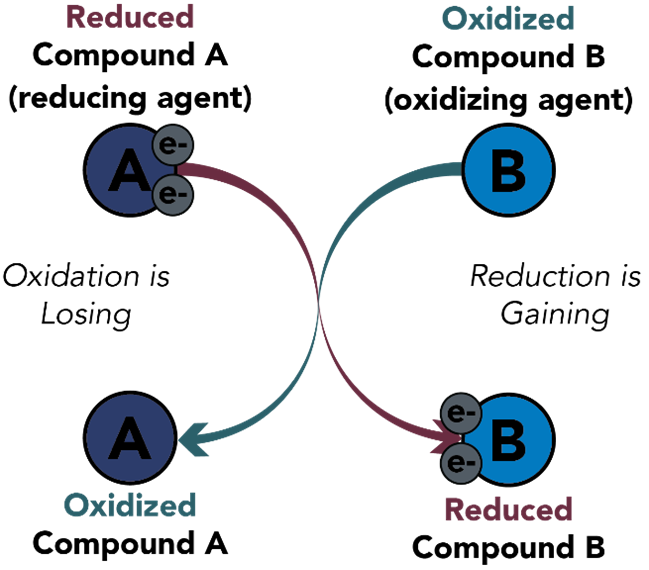
Redox is the movement of electrons. During a redox reaction, electrons are moved from one compound to another. One element or compound is reduced and the other is oxidized (i.e., one compound loses electrons while the other gains).
Figure 1. Redox reaction. this figure shows pairing of oxidation and reduction reactions. The element that is reduced has gained electrons, and the element that is oxidized has lost electrons.
A mnemonic that can be used to remember the direction of electron movement in redox reaction is “OIL RIG”:
Oxidation Is Losing electrons
Reduction Is Gaining electrons
When the ORP values are highly positive, the elements and compounds in the water are in an oxidized state (e.g., NO3-, SO42-, Fe3+, Se6+, etc.). When the ORP values are low, there are more elements and compounds in a reduced state (e.g., NH4+, S2-, Fe2+, Se0, etc.).
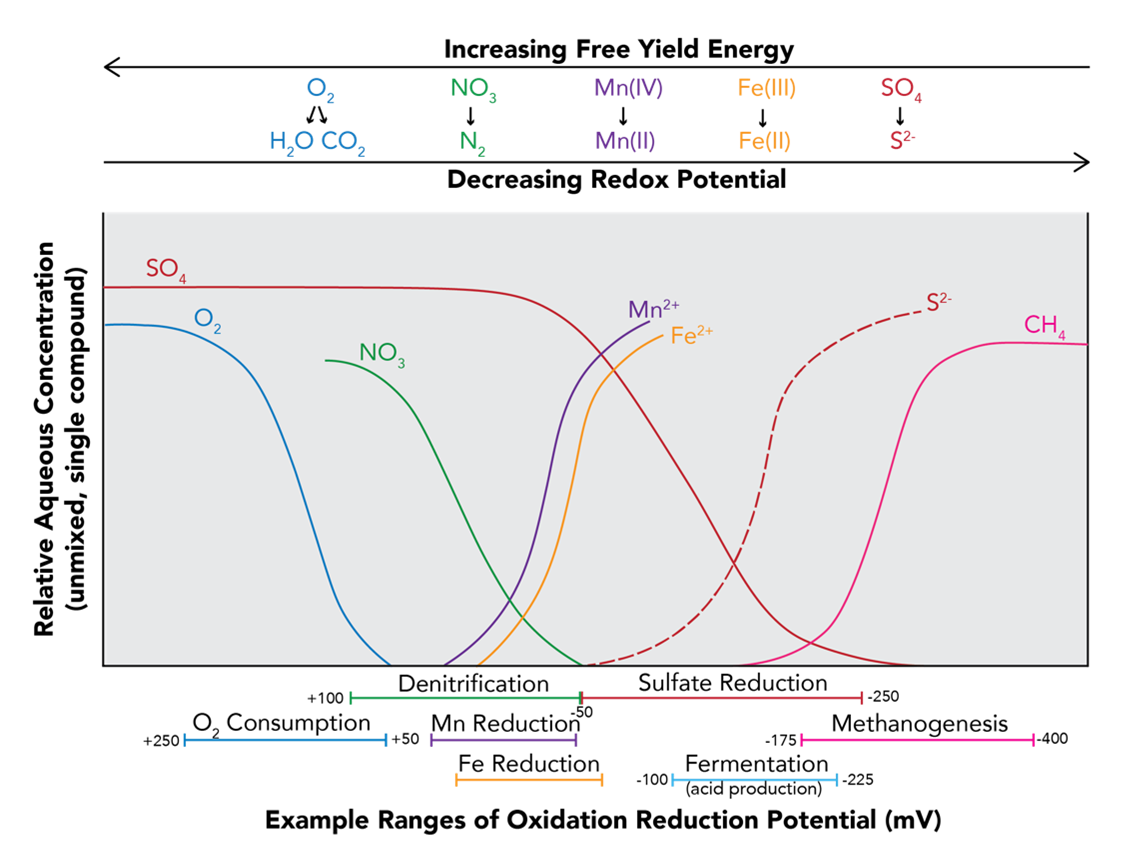
Oxidation-reduction potential (ORP), reported in millivolts (mV), is a measure of the redox status of a water sample. In passive water treatment, we can target the oxidation or reduction of certain constituents to remove them from the water column. The range of redox conditions for particular processes has been well studied, so we can use ORP to understand what reactions will be occurring in a system (Table 2).
Table 2. Examples of ORP ranges for some common biochemical processes
Some elements are easier to oxidize or reduce than others. There is a constant tug-of-war for electrons in solution, and elements that lose the tug-of-war become oxidized. Once all of the strong competitors become reduced (win the electrons), the less competitive species in turn can become reduced by winning remaining electrons against even weaker competitors. Figure 2 shows that sulfate (SO4) is not as strong a competitor for electrons as nitrate (NO3), so nitrate will become reduced before sulfate.
Figure 2. A conceptual example of the order of reactions. Reactions occur in a predictable order. This diagram shows the changes of conceptual relative aqueous concentration of different constituents over a range of redox potentials.